Creating a Document and Change Control Workbook
Training Material Created for a Regulated Biologics Startup
Audience & Context
This workbook was created for a clinical-stage startup focused on the collection and distribution of blood components, operating under FDA and AABB regulations. The team was scaling quickly across multiple sites and needed a standardized way to train staff on change and document control procedures.
It was used by both new hires and cross-functional teams, especially those involved in Quality, Regulatory, Operations, and Manufacturing. The workbook became part of a larger onboarding and compliance training framework.
The Problem
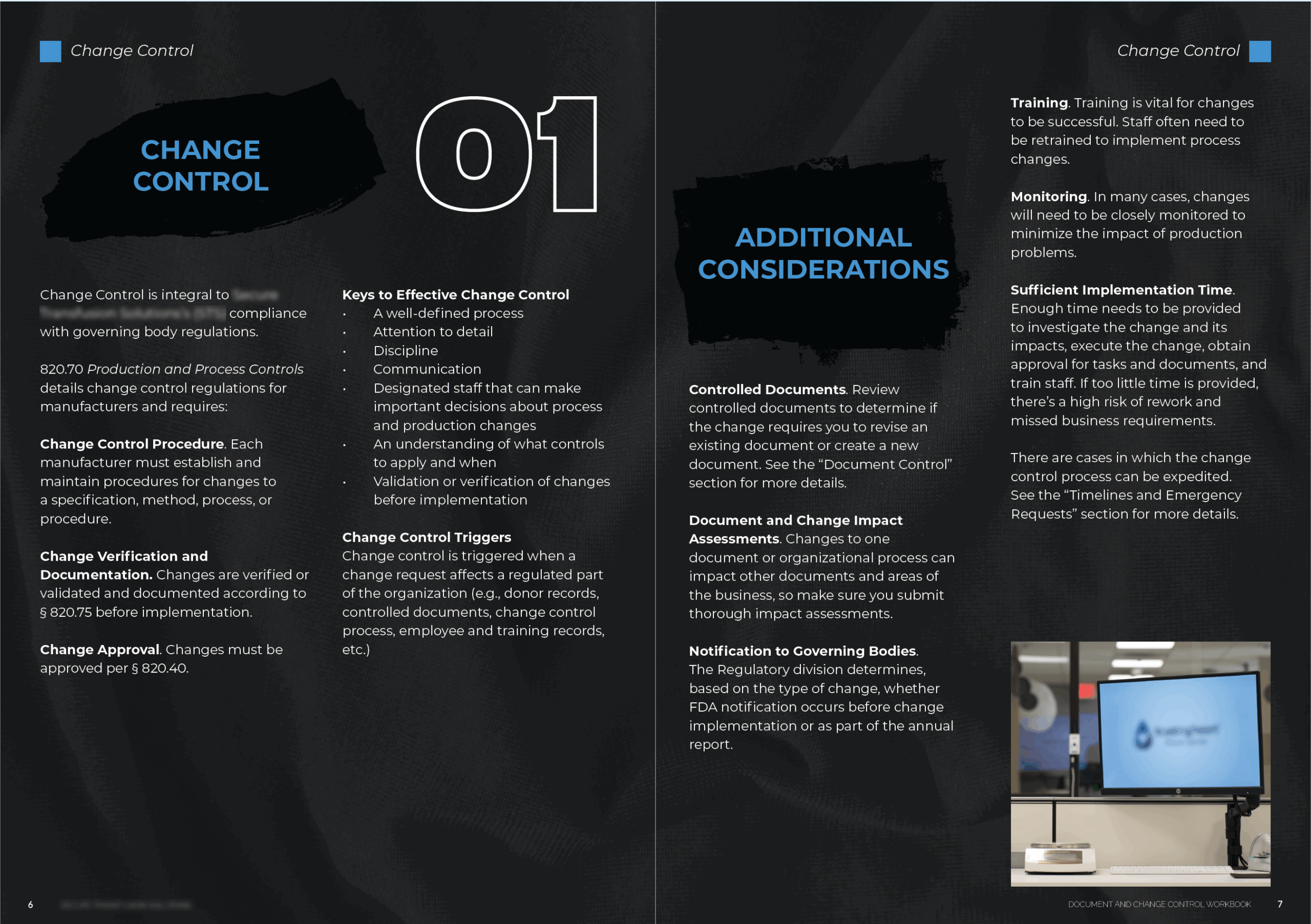
The company was preparing for Biologics License Application (BLA) approval and facing increased regulatory scrutiny. Although SOPs existed, there was no centralized resource that explained the purpose behind change control or connected the dots between regulatory expectations, internal workflows, and submission procedures.
Frontline teams were often unsure what triggered a change control request or how to complete the required documentation. Departments had different understandings of timelines, approvals, and FDA expectations. This led to inconsistent implementation, delays, and audit risk.
The Solution & Process
I developed a print-friendly, comprehensive workbook that explained both change control and document control through the lens of the organization’s internal processes. The project included:
- Breaking down 21 CFR 820.70 and 820.40 into accessible, actionable explanations
- Outlining the internal change control workflow and document control procedures, with embedded links to submission forms and templates
- Creating visual guides and decision trees for departmental impact assessments
- Including annotated examples, sample change requests, and knowledge checks
- Summarizing FDA inspection observations to show real-world consequences of noncompliance
- Organizing timelines, emergency procedures, and escalation protocols in clear language
The content was developed in collaboration with Regulatory, Quality Systems, and Learning and Development teams to ensure it met both compliance and training goals.
The Outcome
The workbook was published internally through SharePoint and used as a reference during onboarding and day-to-day operations. Teams relied on it when submitting change requests or revising controlled documents. Because the content was modular, it was easy to update sections without overhauling the entire resource.
It also helped unify expectations across departments regarding training timelines, required documentation, and who needed to be involved in implementation.

What Success Looks Like
- Consistent documentation practices across teams
- Fewer rejected or incomplete change requests
- More timely submissions aligned with BLA milestones
- Better understanding of training and approval workflows
- Improved audit readiness, backed by examples of FDA expectations and past inspection findings
- Widespread adoption among staff who used the workbook to navigate compliance tasks with confidence


